At the end of February my role on the TW:eed Project came to an end, with the last of the NERC grant funding. I’ll be continuing on in my spare time to finish writing up papers from the project, as will most of the team. In fact it will be hard to keep us away! This project has been brilliant and the most fascinating I have ever worked on, it’s a sad moment to say goodbye.
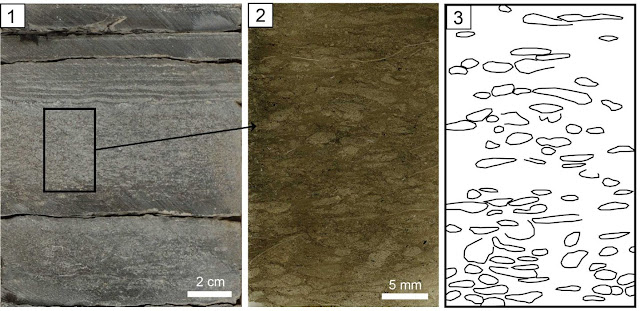
At the beginning of February we held our final team meeting at Cambridge. As always it was fascinating, invigorating and challenging all at once! Research discussed included the latest palynology results from John and Emma, with statistical analysis of the data that is showing some fascinating trends over time. Future efforts by the team will seek to compare the spores found with the macroplant fossils, aiming to track the exact species that produced some of the most common spores, this is not an easy task! Then Tom talked about his latest discoveries working on the lungfish material. Jenny and Tim discussed their latest findings on the tetrapods from our main field site, with new material being discovered in the last few months. I discussed the sedimentological context of tetrapod finds from our study and the broader context. Finally we talked through future plans for a public meeting/symposium and new research project proposals.

|
|
The team of the steps of the Attenborough Building, University Museum of Zoology, Cambridge
|
As a team we have produced so much over the last four years! In total, we have produced 11 published papers, 2 papers in press and 5 that are submitted; 70 published conference abstracts, 64 informal talks, 8 exhibitions, 7 field excursions and workshops; 6 Masters’ theses, 5 undergraduate projects and 32 volunteers at 6th Form and undergraduate levels; 5 informal publications, and numerous social and other media outputs. Wow! A list of our publications can be seen on the tetrapods.org website.
This project has discovered such a huge amount! So much so, that we are still writing up some of our most important findings… we have around 20 papers that are in preparation that the team are working on and hope to publish this year. We really have succeeded with the ‘Team TW:eed’ approach, working well together and learning from each other over the years. I’m glad that I will always have my mug to remind me of this fantastic team!
I want to say a huge thank you to everyone who has supported our project and been involved in research, volunteering, scientific support, educational events and in sharing our work with their friends, family and colleagues. We hope you’ve enjoyed hearing about our discoveries and celebrating Britain’s fossil heritage. Although it’s officially the end…. it’s not the end end…. just try stopping us! I’ll keep updating everyone on the blog with news about our progress and publications throughout 2017.
Until next time
Carys